FHIR Data Integration Process
FHIR
Fast Health Interoperability Resources (FHIR) is a healthcare interoperability standard that defines a content model, in the form of “resources,” and a specification for exchanging the content in real time using RESTful interfaces.
Request Process
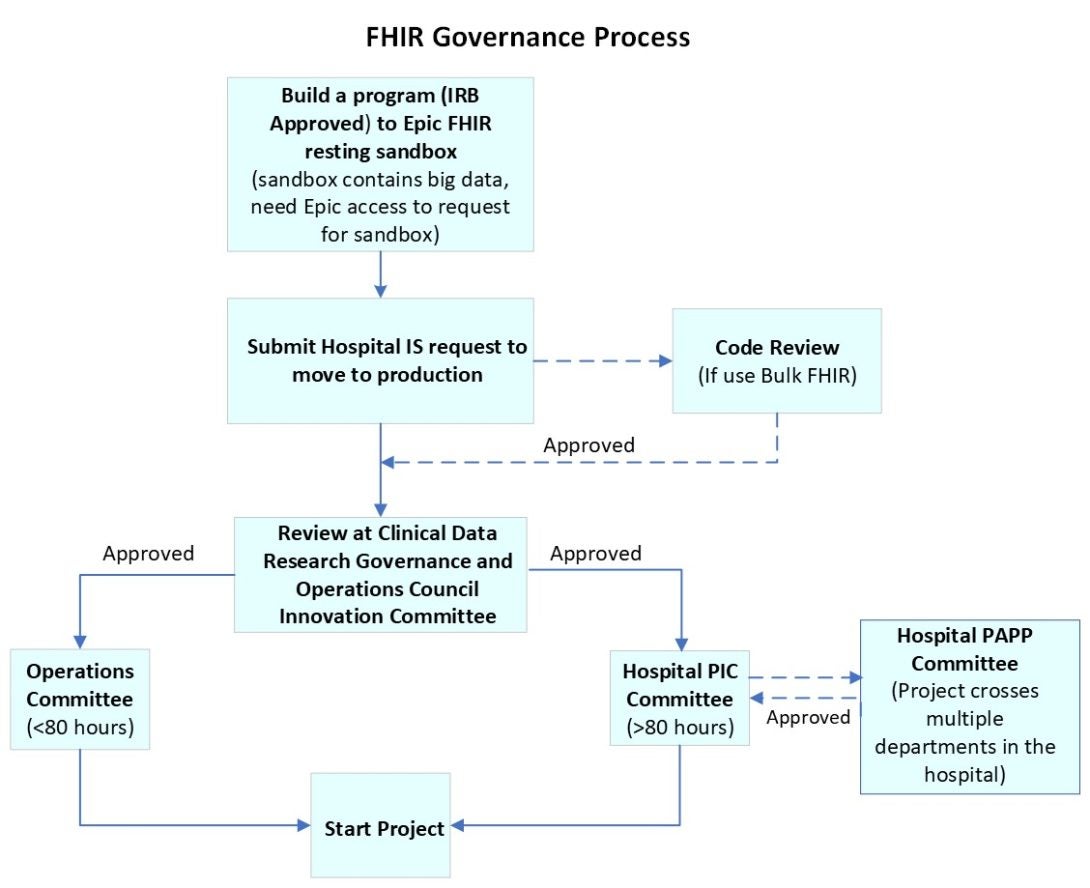
UIC FHIR allows researchers to build a program (IRB approved) to interoperate with the real time Epic health data. Below is the governance process for develop FHIR research applications:
- Build a program to Epic FHIR resting sandbox (test APIs against example data)
- Submit a hospital IS request to move the product to production
- Review at Clinical Data Research Governance and Operations Council Innovation Committee, code review for any Bulk FHIR use
- Go to Clinical Data Research Governance and Operations Council Operations Committee (< 80 hours) or hospital PIC Committee (≥ 80 hours), if the project crosses multiple departments in the hospital, it will go to the hospital PAPP Committee
- Start project
Resources
Epic on FHIR
Epic on FHIR is a free online resource that supports developers who create applications or services for use by provider organizations and wish to interoperate with the Epic comprehensive medical record. At fhir.epic.com, developers will find documentation on Epic’s support for the HL7 FHIR APIs.
Cloud services
- High Performance Computing – This service combines all the computational power of a high-performance computing (HPC) cluster with the convenience and flexibility of the Amazon Web Services (AWS) Cloud. It is a full-featured, on-demand computational cluster consisting of 750 traditional CPU machines, 187 Nvidia GPUs, and six special-purpose high-memory nodes.
- Secure Computing Environment – The Secure Computing Environment (SCE) provides researchers with a platform that mitigates the risks of working with electronic personal health information (ePHI) and other types of high risk data that are subject to regulatory or compliance requirements.