Diversity, Re-Entry, and Re-Integration Supplement Funding
Are you a UIC faculty member looking for ways to recruit and fund postdoctoral researchers? Or are you a trainee considering UIC for your postdoctoral training? Administrative Supplements from the National Institutes of Health (NIH) can support scholars who have identified an NIH-funded lab in which to work. Administrative supplements benefit both postdocs and faculty by providing salary support to outstanding candidates with a quick response turnaround and high funding rate while showing a commitment to a scholar's career by NIH.
Table of Contents Heading link
-
Overview of Administrative Supplements
-
Eligible Faculty and Project Grants
-
Supplement Application and Submission Process
What are Administrative Supplements? Heading link
NIH Administrative Supplements provide additional funding to faculty members and other principal investigators (PIs) who hold active NIH grants (i.e. “parent grants”). Two types of administrative supplements, Diversity Supplements and Re-Entry and Re-Integration Supplements, support trainees by providing funding for postdoctoral training (and other career levels ranging from high school to junior faculty).
Info on Supplements Heading link
NIH Diversity Supplements
Diversity supplements are administrative supplements designed to provide funding support for postdoctoral researchers from diverse backgrounds who wish to participate as researchers in ongoing research projects and career development experiences in preparation for an independent career in health-related research. This experience must serve as a means of assisting the postdoctoral researcher’s development into a productive researcher in health-related science. For more information, see PA-23-189.
Eligible Candidates
- Citizen, non-citizen national or permanent resident of the United States
- Individuals must come from a diverse background, which include:
- Individuals from racial and ethnic groups that have been shown by the NSF to underrepresented in health-related sciences on a national basis: Blacks or African Americans, Hispanics or Latinos, American Indians or Alaska Natives, Native Hawaiians and other Pacific Islanders.
- Individuals with disabilities, who are defined as those with a physical or mental impairment that substantially limits one or more major life activities, as described in the Americans with Disabilities Act of 1990.
- Individuals from disadvantaged backgrounds, defined as those who meet two or more criteria in the Notice of Interest in Diversity (NOT-OD-20-031).
- Individuals typically may only receive one diversity supplements per career level. Some participating NIH Institutes and Centers (ICs) only allow one diversity supplement per individual total, regardless of career stage
- Postdocs who are supported by an Institutional NRSA (T32) or Individual NRSA (F32) are typically eligible once they have completed the grant
- Individuals who are already supported on the applicable parent grant are not typically eligible. In some ICs, an individual may not have any current NIH/PHS support for the proposed career level
Eligible Principal Investigators
- Eligible grant activity codes for the parent grant (PA-23-189):
- DP1, DP2, DP4, DP5
- G12, G20
- P01, P20, P2C, P30, P40, P41, P50, P51, P60, PM1, PN2
- R00, R01, R03, R15, R16, R18, R21, R24, R33, R34, R35, R37, R41, R42, R43, R44, R49, R61, RC1, RC2, RC3, RC4, RF1, RL1, RM1
- SC1, SC2, SC3, S06
- U01, U10, U18, U19, U24, U2C, U34, U41, U42, U44, U54, U56, U60, UC2, UC4, UF1, UG1, UG3, UH2, UH3, UM1, UM2, UL1
- Supplements should support the aims of the parent grant
- Budget requests must not extend beyond the project end date
- Not all participating NIH Institutes and Centers (ICs) support all the activity codes listed above. Each IC also has its own requirements for years remaining on the parent grant to submit a supplement, number of diversity supplement candidates that a parent grant can support, and rules about whether a particular candidate can be supported by multiple diversity supplements. Therefore, before preparing a diversity supplement application, it is IMPERATIVE that the principal investigator reach out to:
- The program officer (PO) for the parent grant
- The institute specific contacts listed here
- Any institute-specific contacts listed in the specific diversity supplement application instructions for that particular IC
Institute Specific Information
The information below is current as of November 1, 2022. However, this information often changes and each IC has its own:
- Submission and funding deadlines
- Application instructions
- Years of funding available for a postdoc candidate
- Eligibility requirements (both for a postdoc candidate and for the PIs parent grant)
- Funding priorities
Therefore, before preparing a diversity supplement application, it is IMPERATIVE that the principal investigator reach out to:
- The program officer (PO) for the parent grant
- The institute specific contacts listed here
- Any institute-specific contacts listed in the specific diversity supplement application instructions for that particular IC
Institute Specific Requirements for Diversity Supplements
| Institute or Center | Minimum Time Remaining on Parent Grant Required | Application Deadlines | Funding Information | Postdoc Support Available |
|---|---|---|---|---|
| National Cancer Institute (NCI) | 2 years remaining at time of application submission | Applications accepted from October 1-December 1 and February 1-March 31 | Funding decisions are anticipated in March and June, respectively | May be supported for the duration of the parent grant, but typically for 3 years or less |
| National Eye Institute (NEI) | Unspecified | Rolling acceptance | ||
| National Heart, Lung, and Blood Institute (NHLBI) | 1 year remaining at time of award | Rolling acceptance | All applications should arrive at least three months before the requested start date. Applications seeking awards before the end of a fiscal year (September 30) must be received no later than May 31 | Supplement may be requested for a minimum of 12 months and a maximum of 4 years. Cumulative postdoctoral research experience must not exceed 6 years inclusive of this supplement award (i.e., Individuals with 2 years prior postdoctoral research training may request up to 4 years of supplement support) |
| National Human Genome Research Institute (NHGRI) | 1 year remaining at time of award | Rolling acceptance | The cutoff to receive an award in the current fiscal year is May 15 | |
| National Institute on Aging (NIA) | 2 years of active status left at the time of supplement application unless the performance period of the grant is less than 2 years | Rolling acceptance | Applications are reviewed within 2-3 months of receipt. Applications seeking consideration before September 30 must be received by May 1 | Maximum length of award is 2 years |
| National Institute on Alcohol Abuse and Alcoholism (NIAAA) | 15 months remaining at time of application submission | Applications are accepted on November 1 and May 1 | Possible award dates are February 1 and August 1 | The minimum duration for a diversity supplement award is 12 months, and up to a maximum of 3 years |
| National Institute of Allergy and Infectious Diseases (NIAID) | 2 years remaining at time of application submission | Rolling acceptance | Applications are reviewed 4 times per year (November, February, April, May) and are awarded 8-10 weeks after review. The cutoff to receive an award in the current fiscal year is April 1 | Duration of support depends on the candidate’s career level and may be adjusted by the review committee |
| National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) | 2 years remaining at time of application submission | Rolling acceptance between October 1 and April 15 | The review process typically takes a minimum 12 weeks | The request cannot exceed the length of time remaining on the parent grant and should be tailored to the postdoc's training plan |
| National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Unspecified | Rolling acceptance | Applications received by May 31 will be reviewed and, if approved for funding, funded in the current fiscal year (by September 30) | The recommended supplement duration is 1-2 years |
| National Institute of Child Health and Human Development (NICHD) | Unspecified | Applications accepted on September 15, January 15, and May 15 | Funding decisions: December, April, and August with funding start dates of January, June, and September | |
| National Institute on Deafness and Other Communication Disorders (NIDCD) | The parent award must have an active budget that is at least equivalent to the duration of the requested supplement | Rolling acceptance | Typically, supplements are provided for 1 year, with a strong justification for a 2nd year | |
| National Institute of Dental and Craniofacial Research (NIDCR) | Unspecified | Rolling acceptance | Reviews take up to 8 weeks. The cutoff to receive an award in the current fiscal year is July 15 | |
| National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | The parent grant must have support remaining for a “reasonable period” (usually 2 years or more) at the time of award | Applications received by 5 pm on the first business day of the month are reviewed at the end of each review month (from October through June) | Funding decisions will be made three times per year in January, April, and June | Maximum length of award is 2 years |
| National Institute on Drug Abuse (NIDA) | 18 months remaining at time of application submission | Rolling acceptance | Applications are reviewed monthly and funding decisions take around 10 weeks following application receipt. The cutoff to receive an award in the current fiscal year is May 9 | Maximum length of award is 2 years |
| National Institute of Environmental Health Sciences (NIEHS) | 2 years remaining at time of application submission | Rolling acceptance | NIEHS reviews supplement applications every other month. The cutoff to receive an award in the current fiscal year is June 1. | Maximum length of award is 2 years |
| National Institute of General Medical Sciences (NIGMS) | The parent grant must have support remaining for a “reasonable period” (usually 2 years or more) at the time of award | Rolling acceptance | Applications are reviewed on a continuous basis. Funding decisions take about 12 weeks. The cutoff to receive an award in the current fiscal year is June | Awards are typically for 2 years. Shorter awards, of at least 12 months, are possible on a case by case basis. If requesting an award after the 3rd year of training or for less than 24 months, contact with NIGMS is required explain the circumstance before submitting an application |
| National Institute of Mental Health (NIMH) | At the time of submission, the proposed supplement activities must be within the approved project period for the parent award. The duration of the proposed supplement should be sufficient in length to provide a meaningful career development experience for the candidate and the duration must be fully justified | Rolling acceptance | Applications are reviewed on a regular schedule. Funding decisions take about 10 weeks. The cutoff to receive an award in the current fiscal year is April 1 | A two-year supplement period is typically appropriate for postdocs. In some circumstances, less than two years might be accommodated, but potential applicants considering a supplement period less than two years would require a detailed explanation |
| National Institute on Minority Health and Health Disparities (NIMHD) | 2 years remaining at time of application submission | Rolling acceptance | Applications are only reviewed twice per year, after November 1 and after June 1, with estimated award start dates of February or September | Maximum length of award is 2 years |
| National Institute of Neurological Disorders and Stroke (NINDS) | The parent grant must have a reasonable period of time remaining (typically, 1-2 years) at the time of possible award to support an optimal career development experience for the candidate | Three application windows per year: November 16-February 15, February 16-May 15, and August 1-November 15 | Funding decisions will be made by the end of March, June, and December, and the earliest expected start date is April, July, and January | Maximum length of award is 2 years |
| National Institute of Nursing Research (NINR) | Unspecified | Applications accepted on January 15, April 15. and August 15 | Funding decisions made within 3 months of application submission | Unspecified, but eligible postdocs must have a degree in nursing |
| National Library of Medicine (NLM) | Unspecified | Rolling acceptance | Applications are reviewed on a continuous basis | |
| John E. Fogarty International Center for Advanced Study in the Health Sciences | Unspecified | Rolling acceptance | Applications are reviewed on a continuous basis | |
| National Center for Complementary and Integrative Health (NCCIH) | Unspecified | Applications are accepted four times a year: January 2nd, April 1st, July 1st, or October 1st | Funding will be provided for 1 year and will consider a 1 year renewal application depending on progress | |
| National Center for Advancing Translational Sciences (NCATS) | 2 years remaining at time of application submission | The submission deadline is November 1 of each year | Applications will be evaluated and decisions will be made within 2-3 months | The requested time should be 2 years |
| Office of Research Infrastructure Programs (ORIP) | 18 months remaining at time of application submission | Rolling acceptance | The proposed start date must be at least 60 days after the date of application submission | The duration of the diversity supplement must be at least 12 months |
| National Institute for Occupational Safety and Health (NIOSH) | Unspecified | Applications accepted between January 1-March 15 |
Re-Entry and Re-Integration Supplements Heading link
Re-Entry and Re-Integration Supplements
According to NOT-OD-21-134, the goal of this program is to provide support for a mentored research training experience for individuals with high potential to re-enter or re-integrate into an active research career, after an interruption for family responsibilities or other qualifying circumstances. It is anticipated that by the completion of the supplement support period, the re-entry/re-integration scientist will be prepared to apply for a fellowship (F), career development (K) award, a research award (R), or other types of independent research support. Applications for this initiative must be submitted using PA-20-272 or its subsequent reissued equivalent. For a list of eligible Activity Codes, see PA-18-592.
Eligible Candidates
- US citizens, non-citizen nationals, or permanent residents
- Must be planning a career in biomedical, behavioral, clinical, translational, or social science research
- Re-entry: In general, the duration of the career interruption should be at least six months for re-entry purposes, and no more than eight years.
- Examples of qualifying interruptions for re-entry supplements include, but are not limited to: a complete or partial hiatus from research activities for child-rearing; an incapacitating illness or injury of the candidate, spouse, partner, or a member of the immediate family; job offers rescinded as a result of natural disasters or public health emergencies (e.g., COVID-19), relocation to accommodate a spouse, partner, or another close family member; pursuit of non-research endeavors that would permit earlier repayment of debt incurred in obtaining a doctoral degree; and military service
- Some ICs require that the duration of the career interruption is at least 1 year
- Re-integration: Candidates seeking to transition out of unsafe research environments because of discriminatory and unlawful harassment are eligible to apply for re-integration supplements as soon as supplement support to continue research training in a new and safe research environment has been identified.
- Candidates may receive support from only one supplement program at a time, but may be supported by more than one supplement during the development of their research careers.
- Some ICs require that candidates must already have a doctoral degree at the time of application
- Some ICs require that candidates must have already been in a postdoctoral position at the time they left active research
Eligible Principal Investigators
- Eligible grant activity codes for the parent grant (PA-18-592):
- DP1, DP2, DP4, DP5
- G12
- P01, P20, P2C, P30, P40, P41, P50, P51, P60, PM1, PN2
- R01, R18, R21, R24, R33, R34, R35, R37, R41, R42, R43, R44, R61, RC1, RC2, RC3, RC4, RF1, RM1
- S06
- U01, U10, U18, U19, U24, U2C, U41, U42, U44, U54, U56, UC2, UF1 UG1, UG3, UH2, UH3, UM1, UM2
- The P20, P30, and P60 award mechanisms are eligible for supplements only if they contain research components
- Supplements should support the aims of the parent grant
- Budget requests must not extend beyond the project end date and must fall within the budget cycle of the parent grant
- A parent grant may support only one individual on a supplement
- Not all participating NIH Institutes and Centers (ICs) support all the activity codes listed above. Each IC also has its own requirements for years remaining on the parent grant to submit a supplement and other eligibility requirements. Therefore, before preparing a re-entry or re-integration supplement application, it is IMPERATIVE that the principal investigator reach out to:
- The institute specific contacts listed here
- The program officer (PO) for the parent grant
- Any institute-specific contacts listed in the specific supplement application instructions for that particular IC
Institute Specific Information
The information below is current as of November 1, 2022. However, this information often changes and each IC has its own:
- Submission and funding deadlines
- Application instructions
- Years of funding available for a postdoc candidate
- Eligibility requirements (both for a postdoc candidate and for the PIs parent grant)
- Funding priorities
Therefore, before preparing a diversity supplement application, it is IMPERATIVE that the principal investigator reach out to:
- The institute specific contacts listed here
- The program officer (PO) for the parent grant
- Any IC-specific contacts listed in the specific supplement application instructions for that particular IC
Institute Specific Information
| Institute Specific Information | Minimum Time Remaining on Parent Grant Required | Application Deadlines | Funding Information | Postdoc Support Available |
|---|---|---|---|---|
| National Cancer Institute (NCI) | 2 years remaining at the time of application submission | Applications accepted October 1-December 1 and February 1-March 31 | Funding decisions are anticipated in March and June, respectively | May be supported for the duration of the parent grant, but typically for 3 years or less |
| National Eye Institute (NEI) | Unspecified | Rolling acceptance | ||
| National Heart, Lung, and Blood Institute (NHLBI) | 1 year remaining at time of award | Rolling acceptance | All applications should arrive at least three months before the requested start date. Applications seeking awards before the end of a fiscal year (September 30) must be received no later than May 31 | A minimum of 1 year and a maximum of 3 years of support will be awarded |
| National Human Genome Research Institute (NHGRI) | 1 year remaining at time of award | Rolling acceptance | Administrative supplements may be submitted throughout the fiscal year, but should be requested at least 90 days prior to the anticipated need and no later than by May 15 | |
| National Institute on Aging (NIA) | 2 years of active status left at the time of supplement application unless the performance period of the grant is less than 2 years | Rolling acceptance | 2 years of active status left at the time of supplement application unless the performance period of the grant is less than 2 years | Maximum length of award is 2 years |
| National Institute on Alcohol Abuse and Alcoholism (NIAAA) | Unspecified | |||
| National Institute of Allergy and Infectious Diseases (NIAID) | 2 years remaining at time of application submission | Rolling acceptance | Applications are reviewed 4 times per year (November, February, April, May) and are awarded 8-10 weeks after review. The cutoff to receive an award in the current fiscal year is April 1 | |
| National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) | Unspecified | Rolling acceptance between October 1 and May 31 | ||
| National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Unspecified | |||
| National Institute of Child Health and Human Development (NICHD) | Unspecified | Applications accepted on September 15, January 15, and May 15 | Funding decisions: December, April, and August with funding start dates of January, June, and September | |
| National Institute on Deafness and Other Communication Disorders (NIDCD) | Unspecified | Rolling acceptance | Minimum length of award is 1 year | |
| National Institute of Dental and Craniofacial Research (NIDCR) | Unspecified | Rolling acceptance | Applications are reviewed on a continuous basis. The cutoff for current fiscal year funding is July 15 | |
| National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | The parent grant must have support remaining for a “reasonable period” (usually 2 years or more) at the time of award | Applications received by 5 pm on the first business day of the month are reviewed at the end of each review month (from October through June) | Funding decisions will be made three times per year in January, April, and June | Maximum length of award is 2 years |
| National Institute on Drug Abuse (NIDA) | Unspecified | |||
| National Institute of Environmental Health Sciences (NIEHS) | The parent grant must have a reasonable period of research support (usually 2 years or more) remaining at the time of the supplemental award | Rolling acceptance | Re-entry supplement applications are reviewed every other month to minimize the time from application to award. The cutoff to receive an award in the current fiscal year is June 1 | |
| National Institute of General Medical Sciences (NIGMS) | The parent grant must have a reasonable period of research support remaining at the time of the supplemental award | Rolling acceptance, but applications submitted after May 1 may not be awarded until the following November due to the change in fiscal year on October 1 | Funding decisions take approximately 12 weeks following receipt of the application | Maximum length of award is 3 years |
| National Institute of Mental Health (NIMH) | At the time of submission, the proposed supplement activities must be within the approved project period for the parent award. The duration of the proposed supplement should be sufficient in length to provide a meaningful career development experience for the candidate and the duration must be fully justified | Rolling acceptance | Applications are reviewed on a regular schedule. Funding decisions take about 10 weeks. The cutoff to receive an award in the current fiscal year is April 1 | A two-year supplement period is typically appropriate for postdocs. In some circumstances, less than two years might be accommodated, but potential applicants considering a supplement period less than two years would require a detailed explanation |
| National Institute on Minority Health and Health Disparities (NIMHD) | Unspecified | |||
| National Institute of Neurological Disorders and Stroke (NINDS) | 1 year remaining at the time of award | Rolling acceptance | Funding decisions take 1-2 months following application submission | Minimum length of award is 1 year, maximum length is 3 years |
| National Institute of Nursing Research (NINR) | Unspecified | |||
| National Library of Medicine (NLM) | Unspecified | Rolling acceptance | ||
| John E. Fogarty International Center for Advanced Study in the Health Sciences | Unspecified | |||
| National Center for Complementary and Integrative Health (NCCIH) | Unspecified | Applications are accepted four times a year: January 2nd, April 1st, July 1st, or October 1st | Funding will be provided for 1 year and will consider a 1 year renewal application depending on progress | |
| National Center for Advancing Translational Sciences (NCATS) | 2 years remaining at the time of application submission | The submission deadline is November 1 of each year | Applications will be evaluated and decisions will be made within 2-3 months | The requested time should be 2 years |
| Office of Research Infrastructure Programs (ORIP) | Unspecified | |||
| Office of Research on Women’s Health (ORWH) | Unspecified | Minimum length of award is 1 year | ||
| NIH Common Fund | Unspecified |
Eligible Investigator/Project Database Heading link
The following faculty members have active NIH parent grants that are potentially eligible to receive Administrative Supplement funding from NIH. These faculty members have indicated their interest in recruiting postdocs through this supplement mechanism. Eligible prospective postdocs, please read through the database of projects and contact any faculty members of interest directly. To learn more about a potential project, you can:
- Download this sortable, searchable Excel file of projects with project abstracts
- Search the project award number in the NIH RePORTER database
Postdoc Supplement Eligible PI Database Heading link
| PI Name | PI Email | UIC Department | Project Title | NIH IC | Project Award Number | Project End Date |
|---|---|---|---|---|---|---|
| Alonzo, Francis | falonzo@uic.edu | Microbiology & Immunology | Intercellular Communication and Pheromone Maturation in Gram-Positive Bacteria | NIAID | R01 AI153059 | April 30, 2025 |
| Alonzo, Francis | falonzo@uic.edu | Microbiology & Immunology | Staphylococcus aureus Survival During Nutrient Restriction and Suppression of Host Immunity | NIAID | R01 AI120994 | February 28, 2026 |
| Alsberg, Eben | ealsberg@uic.edu | Biomedical Engineering | Individual cell bioprinting to generate multi-tissue type condensations for osteochondral tissue regeneration | NIAMS | R01 AR081448 | February 29, 2028 |
| Argos, Maria | argos@uic.edu | Epidemiology and Biostatistics | Impact of Metals on Biological Aging and Cardiometabolic Traits in Adolescents | NIEHS | R01 ES033883 | March 31, 2027 |
| Bhatt, Tanvi | tbhatt6@uic.edu | Physical Therapy | Neuromechanisms of falls in older adults with MCI: Targeting assessment and training of reactive balance control | NIA | R01 AG073152 | August 31, 2026 |
| Bronas, Ulf | bronas@uic.edu | Behavioral Nursing Science | Accelerated Age-related Cognitive Decline: Impact of Exercise on Executive Function and Neuroplasticity (EXEC-study) | NIA | R01 AG076456 | April 30, 2027 |
| Caskey, Rachel | rcaskey@uic.edu | Medicine-Academic Internal Medicine | Improving Maternal Health Through an Adaptation of a Two-Generation Postpartum Care Model in Diverse Settings | NICHD | R21 HD112104 | August 31, 2024 |
| Coloff, Jonathan | coloff@uic.edu | Physiology & Biophysics | Targeting Serine Auxotrophy in Luminal Breast Cancer | NCI | R37 CA251216 | February 28, 2026 |
| Cologna, Stephanie | cologna@uic.edu | Chemistry | Novel Therapeutic Approaches for NPC Disease | NINDS | R01 NS124784 | June 30, 2027 |
| Cordoba-Chacon, Jose | jcordoba@uic.edu | Medicine-Endocrinology/Diabetes & Metabolism | PPARgamma-regulated mechanisms in hepatocytes that promote NAFLD | NIDDK | R01 DK131038 | January 31, 2027 |
| DiPietro, Luisa | ldipiet@uic.edu | Periodontics | Complexity and the Wound Healing Response | NIGMS | R35 GM139603 | December 31, 2025 |
| Djalilian, Ali | adjalili@uic.edu | Opthlamology & Visual Science | Phase I Study of Mesenchymal Stromal Cell Secretome for Promoting Corneal Regeneration | NEI | UH3 EY031809 | September 9, 2024 |
| Dykens, Andrew | jdykens@uic.edu | Family & Community Medicine | Adaptation and implementation of a patient navigation program for cervical cancer screening across contexts in Senegal | NCI | R01 CA258683 | March 31, 2027 |
| Er, Emrah | eer@uic.edu | Physiology & Biophysics | Microsurveillance in Breast Cancer Metastasis | NCI | R37 CA269370 | August 31, 2027 |
| Federle, Michael | mfederle@uic.edu | Pharmaceutical Sciences | Macrophage Immunosuppression by Quorum-Induced Streptococcus pyogenes | NIAID | R01 AI162679 | June 30, 2026 |
| Fukuchi, Ken-ichiro | kfukuchi@uic.edu | Cancer Biology & Pharmacology | Role of MyD88 signaling in systemic inflammation and Alzheimer disease | NIA | R01 AG069447 | April 30, 2026 |
| George, Anne | anneg@uic.edu | Oral Biology | Role of DMP1 Mediated Paracrine Signaling in Vasculogenesis | NIDCR | R01 DE031737 | June 30, 2027 |
| Gowrishankar, Swetha | swethag@uic.edu | Anatomy & Cell Biology | Elucidating the role of Adaptor Protein complex-4 in regulating axonal autophagic and lysosomal pathways | NIA | R01 AG074248 | May 31, 2027 |
| Hay, Nissim | nhay@uic.edu | Biochemistry & Molecular Genetics | Hexokinase 2 and cancer therapy | NCI | R01 CA258299 | June 30, 2026 |
| He, Bin | tshuo@uic.edu | Microbiology & Immunology | Viral determinants in HSV virulence | NIAID | R01 AI148148 | April 30, 2025 |
| Hu, Guochang | gchu@uic.edu | Anesthesiology | Targeting the host immune response during sepsis | NHLBI | R01 HL152696 | June 30, 2024 |
| Hu, Samuel | yshu@uic.edu | Chemistry | Ultrasensitive quantification of cytokine release from T cells | NIGMS | R35 GM146786 | May 31, 2027 |
| Kazlauskas, Andrius | ak20@uic.edu | Opthlamology & Visual Science | Anti-VEGF-mediated barrier closure | NEI | R01 EY031350 | August 31, 2025 |
| Kuchay, Shafi | kuchay@uic.edu | Biochemistry & Molecular Genetics | Proteostasis at cellular membranes | NIGMS | R35 GM137452 | July 31, 2025 |
| Lash, James | jplash@uic.edu | Medicine-Nephrology | University of Illinois at Chicago KPMP CKD Recruitment Site | NIDDK | U01 DK133081 | June 30, 2027 |
| Lash, James | jplash@uic.edu | Medicine-Nephrology | Chronic Renal Insufficiency Cohort Study | NIDDK | U01 DK060980 | June 30, 2023 |
| Lash, James | jplash@uic.edu | Medicine-Nephrology | Chicago Kidney Urology Hematology Network for Citywide Research Training and Career Development | NIDDK | U2C DK129917 | May 31, 2026 |
| Lazarov, Orly | olazarov@uic.edu | Anatomy & Cell Biology | Hippocampal neurogenesis in cognitive function and dysfunction in Alzheimer's disease | NIA | R01 AG076940 | April 30, 2027 |
| Lazarov, Orly | olazarov@uic.edu | Anatomy & Cell Biology | The role of APP in neurogenesis and AD in Down syndrome | NIA | RF1 AG079002 | August 31, 2025 |
| Lee, Steve | ssylee@uic.edu | Pharmaceutical Sciences | Integrated three-dimensional (3D) microscopy for a spatial pharmacology atlas of macromolecular drugs in the tissue microenvironment | NIGMS | R35 GM142743 | August 31, 2026 |
| Li, Hongjin | hongjin@uic.edu | Human Development Nursing Science | Feasibility of Implementing Acupuncture into a Federally Qualified Health Center to Alleviate Multiple Symptoms Among Breast Cancer Survivors Receiving Endocrine therapy | NCCIH | R34 AT012084 | May 31, 2025 |
| Ma, Ao | aoma@uic.edu | Biomedical Engineering | Understanding allostery from the perspective of protein dynamics and energy flows | NIAID | R21 AI162197 | July 31, 2024 |
| Macduff, Donna | dmacduff@uic.edu | Microbiology & Immunology | Defining the differential roles of HOIL1 and the Linear Ubiquitin Chain Assembly Complex in interferon induction by MDA5 and RIG-I during viral infection | NIAID | R01 AI150640 | May 31, 2026 |
| Madhavan, Sangeetha | smadhava@uic.edu | Physical Therapy | Cortical priming to optimize gait rehabilitation post stroke | NICHD | R01 HD075777 | July 31, 2025 |
| Mankad, Neal | npm@uic.edu | Chemistry | Multimetallic Catalysis in Biology and Synthesis | NIGMS | R35 GM140850 | May 31, 2026 |
| Mankin, Alexander | shura@uic.edu | Pharmaceutical Sciences | Advancing ribosome-targeting antibacterial peptides with a unique mechanism of action | NIAID | R01 AI162961 | January 31, 2027 |
| Marai, Georgeta-Elisabeta | gmarai@uic.edu | Computer Science | Longitudinal Spatial-Nonspatial Decision Support for Competing Outcomes in Head and Neck Cancer Therapy | NCI | R01 CA258827 | February 28, 2026 |
| Martin, Molly | mollyma@uic.edu | Pediatrics | Coordinated Oral Health Promotion (CO-OP) Chicago Cohort Study | NIDCR | U01 DE030067 | June 30, 2026 |
| Mehta, Tara | tmehta@uic.edu | Psychiatry | Patient Navigators for Children's Community Mental Health Services in High Poverty Urban Communities | NIMH | R01 MH123424 | February 28, 2026 |
| Mermelstein, Robin | robinm@uic.edu | Psychology | Context, Subjective and Cognitive Experiences with Patterns of Tobacco and Cannabis Co-Use in Young Adults | NIDA | R01 DA051157 | April 30, 2025 |
| Mermelstein, Robin | robinm@uic.edu | Center for Clinical and Translational Science | Clinical and Translational Science Award | NCATS | UL1 TR002003 | May 31, 2025 |
| Mo, Gary | gmo@uic.edu | Pharmacology & Regenerative Medicine | Pyroptosis is a Trial-by-Fire Program | NIGMS | R35 GM146936 | July 31, 2027 |
| Naba, Alexandra | anaba@uic.edu | Physiology & Biophysics | Thinking outside the cell: Leveraging HuBMAP data to build the human ECM atlas | NHGRI | U01 HG012680 | April 30, 2026 |
| Naba, Alexandra | anaba@uic.edu | Physiology & Biophysics | Enhanced mass-spectrometry-based approaches for in-depth profiling of the cancer extracellular matrix | NCI | R21 CA261642 | August 31, 2025 |
| Nakamura, Toru | nakamut@uic.edu | Biochemistry & Molecular Genetics | Regulation of Telomere Maintenance in Fission Yeast | NIGMS | R01 GM143316 | August 31, 2026 |
| Ong, Sang Ging | sangging@uic.edu | Pharmacology & Regenerative Medicine | Rab GTPases-mediated mitochondrial clearance in diabetic cardiomyopathy | NHLBI | R01 HL148756 | March 31, 2025 |
| Reed, David | reedd@uic.edu | Oral Biology | Cell-Matrix Regulation of Fibrochondrocytes in TMJ OA | NIDCR | R01 DE029835 | March 31, 2025 |
| Richner, Justin | richner@uic.edu | Microbiology & Immunology | Dengue virus mRNA lipid nanoparticle vaccine | NIAID | R01 AI150672 | July 31, 2025 |
| Riley, Andrew | apriley@uic.edu | Pharmaceutical Sciences | Synthesis and Evaluation of Alkaloids to Probe Membrane Receptors | NIGMS | R35 GM147005 | June 30, 2027 |
| Roitman, Mitchell | mroitman@uic.edu | Psychology | Modulation of Nac-DA Signaling by Learning, Motivational State and Peptides | NIDA | R01 DA025634 | June 30, 2025 |
| Rong, Lijun | lijun@uic.edu | Microbiology & Immunology | Optimizing Ridaifen-B analogs as potential therapeutics for Ebola viruses | NIAID | R01 AI168362 | July 31, 2027 |
| Ross, Susan | srross@uic.edu | Microbiology & Immunology | The role of TRIM2 and SIRPA in New World Arenavirus entry | NIAID | R01 AI159290 | April 30, 2027 |
| Roth, Steven | rothgas@uic.edu | Anesthesiology | VRC: Engineered extracellular vesicles for mild TBI-induced retinal injury | NEI | R01 EY034716 | May 31, 2025 |
| Roth, Steven | rothgas@uic.edu | Anesthesiology | Mesenchymal stem cell extracellular vesicles for ischemic retinal damage | NEI | R01 EY033902 | May 31, 2026 |
| Salles, Angeles | salles@uic.edu | Biological Sciences | Neural processing of communication sounds: acoustic features and semantic content | NIDCD | R00 DC019145 | July 31, 2025 |
| Santosh, Saraf | ssaraf@uic.edu | Medicine-Hematology & Oncology | Pathways of Cell-Free Hemoglobin in Sickle Cell Nephropathy | NHLBI | R01 HL153161 | June 30, 2025 |
| Shahrara, Shiva | shahrara@uic.edu | Medicine-Rheumatology | Identifying a novel pathway that regulates RA immunometabolism | NIAID | R01 AI167155 | June 30, 2027 |
| Shukla, Deepak | dshukla@uic.edu | Opthlamology & Visual Science | HSV-1 Encoded MicroRNAs in the Pathogenesis and Treatment of Ocular Herpes | NEI | R01 EY033622 | December 31, 2025 |
| Shukla, Deepak | dshukla@uic.edu | Opthlamology & Visual Science | Core Grant for Vision Research | NEI | P30 EY001792 | August 31, 2026 |
| Shukla, Deepak | dshukla@uic.edu | Opthlamology & Visual Science | Alleviation of ER stress as a translational strategy to curb ocular viral infections | NEI | R24 EY033598 | December 31, 2026 |
| Song, Zhenyuan | song2008@uic.edu | Kinesiology & Nutrition | Hepatic Nicotinamide N-Methyltransferase (NNMT) as a Pathogenetic Mechanism and Therapeutic Target for Alcoholic Liver Disease | NIAAA | R01 AA030255 | May 31, 2027 |
| Stocco, Carlos | costocco@uic.edu | Physiology & Biophysics | Salt-Inducible Kinase Regulation of Ovarian Granulosa Cells | NICHD | R01 HD097202 | May 31, 2024 |
| Tiruppathi, Chinnaswamy | tiruc@uic.edu | Pharmacology & Regenerative Medicine | Transcription Factor Elf2 Signals Resolution of Lung Injury | NHLBI | R01 HL156965 | February 28, 2025 |
| Wang, Jim | zjwang@uic.edu | Pharmaceutical Sciences | Molecular mechanism and targeting of chronic pain in sickle cell disease | NHLBI | R35 HL140031 | December 31, 2024 |
| Wang, Xiaowei | xwang317@uic.edu | Pharmacology & Regenerative Medicine | Combined Computational and Experimental Analyses of Gene Regulation by MicroRNAs | NIGMS | R35 GM141535 | May 31, 2026 |
| Xu, Pingwen | pingwenx@uic.edu | Medicine-Endocrinology/Diabetes & Metabolism | Testosterone and estrogen signaling pathways in the medial amygdala interact to control energy homeostasis | NIDDK | R01 DK123098 | June 30, 2025 |
| Zak, Joseph | jdzak@uic.edu | Biological Sciences | Learning-mediated plasticity in cortical feedback projections to the olfactory bulb | NIDCD | R00 DC017754 | February 28, 2025 |
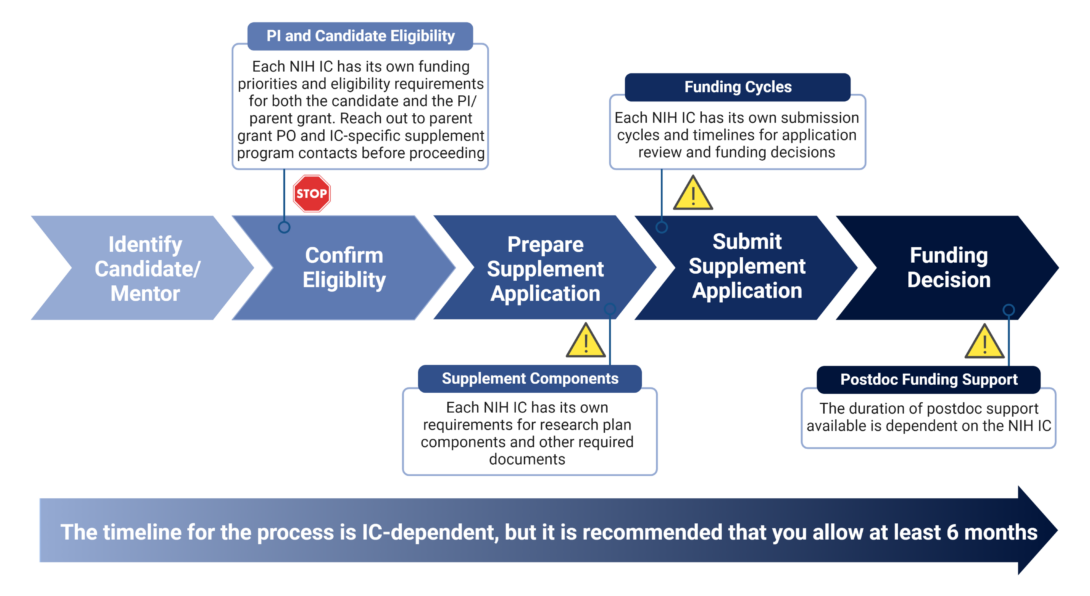
Supplement Preparation Process Heading link
After identifying a candidate/mentor PI pair, it is imperative that the PI reach out to the PO of the parent grant and any IC-specific contacts associated with the appropriate administrative supplement program in that IC to confirm eligibility of the parent grant and candidate, confirm funding priorities of the IC, and confirm IC-specific submission components and deadlines. The candidate and PI will then work together to prepare and submit the supplement application.
Supplement Submission Components Heading link
Before getting started, review the specific requirements for your IC and reach out to the program officer (PO) for the parent grant as well as any institute-specific contacts listed in the specific supplement application instructions for that particular IC. Additional information on submitting administrative supplements can be found here.
It is critical that applicants follow the instructions for their submission option (SF424 (R&R) Application Guide) except where instructed in funding opportunity announcement:
- Diversity Supplement FOA (PA-23-189)
- Notice of NIH’s Interest in Diversity (NOT-OD-20-031)
- Re-Entry and Reintegration FOA (PA-20-272)
- Notice of Special Interest (NOSI): Research Supplements to Promote Re-Entry and Re-integration into Health-Related Research Careers (NOT-OD-21-134)
- List of eligible Activity Codes (PA-18-592)
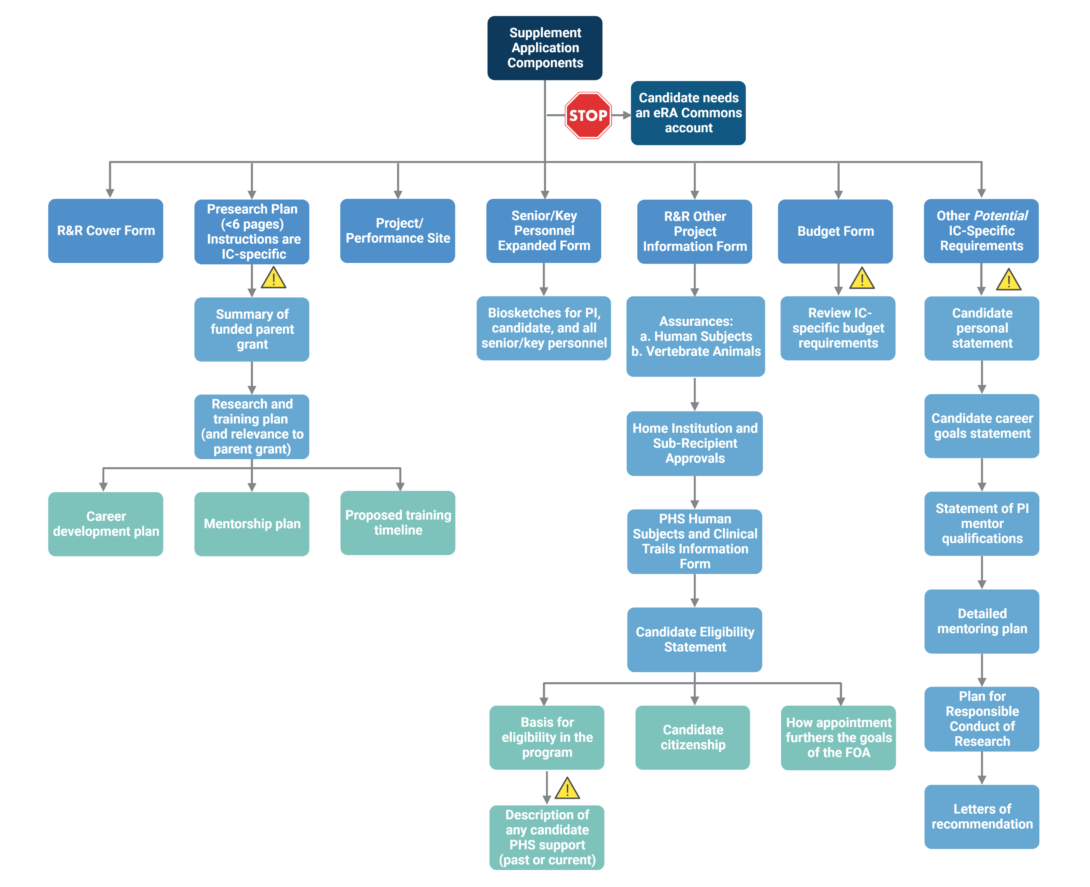
The flowchart below depicts the components typically included in a supplement submission.
The main component of the supplement submission is the research plan, which is 6 pages or less. Click here for an example of a funded postdoc diversity supplement research plan from the University of Washington.
Pay close attention to IC-specific instructions for preparing the supplement application, as each IC has its own requirements. Components that may have IC-specific instructions are indicated by the yellow triangles.